Corrosion and Material Selection in Seawater Scenarios
Author: Date:2025-02-14 Browse Volume:100 timesRecently, I came across a piece of news. A netizen drove their car to the seaside to "wash it", but not only did the car not get clean, but it couldn't even move forward. After some attempts, the car owner finally had to seek help from a towing company for rescue, thus becoming a negative example of "washing a car with seawater". Car enthusiasts all hope that their vehicles are always shiny and beautiful. But do you know that seawater is definitely not a good choice for washing cars.

Washing a Car with Seawater — A "Nightmare" for Cars Seawater is full of salt, accounting for approximately 3.5%. Saline substances such as sodium chloride and magnesium chloride are simply the "natural enemies" of car paint and metal components. Once a car is washed with seawater, these salts will quickly start the "destruction mode", reacting chemically with the car paint, making the originally shiny car paint gradually become dull. Over time, it will peel off and fall off, and the appearance of the car will instantly deteriorate. For the car chassis, it is even worse. The chassis is exposed for a long time. After the seawater salts adhere to it, they will corrode the metal structure of the chassis, greatly reducing its strength and stability. It's just like burying a hidden danger in the "foundation" of the car, and the driving safety may be threatened at any time. Moreover, there are far more impurities in seawater than one can imagine, including microorganisms, algae, and sediment. When washing a car with seawater, these impurities will run around with the water flow and invade key parts of the car, such as the engine compartment, radiator, and air conditioning system. Just imagine, if the engine compartment is blocked by sediment, the heat dissipation will be poor, and the engine is very likely to overheat or even "stop working"; if the air conditioning system is occupied by microorganisms, the cooling and heating effects will be completely lost; if impurities get into the braking system, the braking sensitivity will decrease and the braking distance will increase. How can driving safety be guaranteed? In addition, the weak alkalinity of seawater will erode the rubber of the tires, making the tires hard and brittle, shortening their service life. It will also affect the handling performance due to uneven friction. Driving will be like walking on a tightrope, full of dangerous situations.
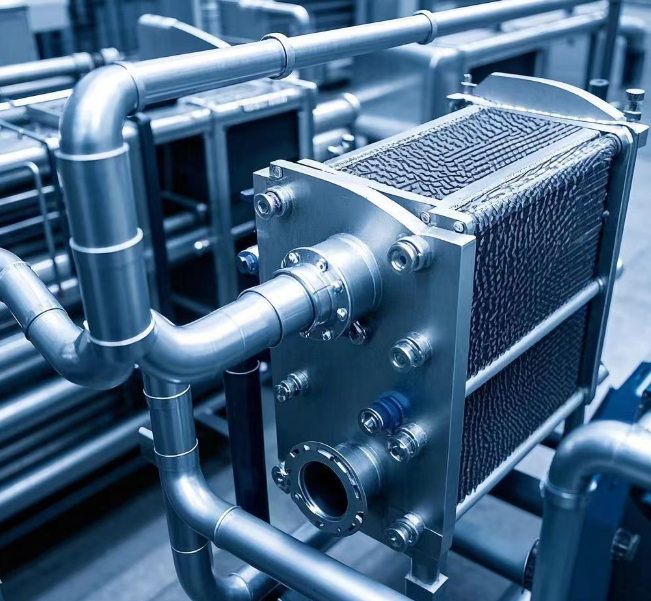
Material Selection for Heat Exchangers in Mariculture and Seawater Desalination From the "dilemma" of cars to the fields of mariculture and seawater desalination related to me, the selection of materials for the key equipment here — plate heat exchangers, is really particular.
For plate materials, titanium and titanium alloys can be regarded as "star players". In seawater, titanium can easily resist the erosion of chloride ions with its excellent corrosion resistance, without rusting or pitting, and steadily ensuring the long-term and efficient operation of the heat exchanger. At the same time, it has high strength and low weight. It can withstand the pressure of seawater without adding too much burden to the overall equipment. Industrial pure titanium such as TA1 is often the first choice. The 316L type of stainless steel, due to its relatively high molybdenum content, also has strong corrosion resistance to seawater and has a relatively affordable price. It is widely used in some mariculture scenarios where the cost control is strict and the corrosiveness of seawater is slightly weaker.
For example, Hastelloy is a high-end choice. In the face of harsh seawater environments such as high temperature and high salinity, it can calmly deal with them and has extremely high chemical tolerance. However, the cost is relatively high, and it mostly shines in top-level mariculture projects with extremely high requirements for corrosion resistance. The gasket material is also crucial, just like the tires of a car. Nitrile rubber emerges with good oil resistance, wear resistance, and anti-aging properties. It has good tolerance to seawater, can achieve a perfect seal within a certain temperature range, has sufficient elasticity, and has good cost-effectiveness. It is a common choice for gaskets of plate heat exchangers in mariculture.
Ethylene propylene diene monomer (EPDM) rubber stands out with its excellent weather resistance, water resistance, and chemical corrosion resistance. It is not easy to age or be damaged even when soaked in seawater for a long time, and its sealing performance is always good. It is especially suitable for occasions with dual high standards of corrosion resistance and aging resistance. In short, whether it is to protect cars from the damage of seawater or to select suitable heat exchanger materials for mariculture and seedling breeding, understanding the characteristics of seawater and making correct choices accordingly are the keys to ensuring performance and extending service life. I hope today's sharing can be beneficial to everyone, enabling you to avoid the "seawater minefields" in your respective fields and move towards success!
The differences between genuine imported products and domestically produced narrow metal-rolled strips.
Next:Hastelloy “BC-1®”




